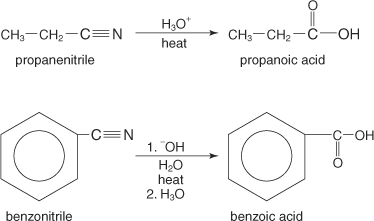
Hydrolysis of nitriles
The hydrolysis of nitriles, which are organic molecules containing a cyano group, leads to carboxylic acid formation. These hydrolysis reactions can take place in either acidic or basic solutions.
|
The mechanism for these reactions involves the formation of an amide followed by hydrolysis of the amide to the acid. The mechanism follows these steps:
-
The nitrogen atom of the nitrile group is protonated.

-
The carbocation generated in Step 1 attracts a water molecule.
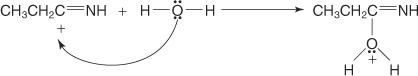
-
The oxonium ion loses a proton to the nitrogen atom, forming an enol.
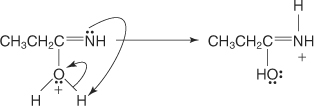
-
The enol tautomerizes to the more stable keto form.
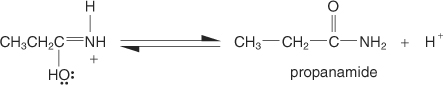
-
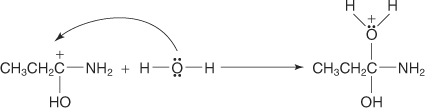
The amide is protonated by the acid, forming a carbocation.
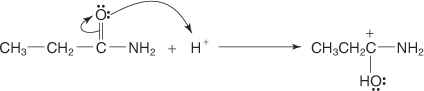
-
A water molecule is attracted to the carbocation.
-
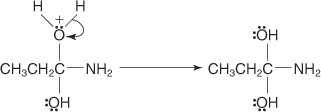
The oxonium ion loses a proton.
-
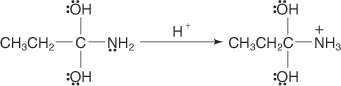
The amine group is protonated.
-
An electron pair on one of the oxygens displaces the ammonium group from the molecule.

|
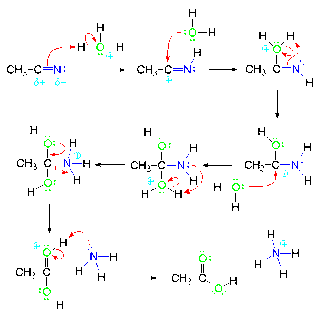
Nitrile Hydrolysis under Acidic Conditions
Nitrile Hydrolysis under Basic Conditions
Grignard Reaction of Nitriles


Hi Yuli.... I am thankful to you, you've post about reaction mecanism of Hydrolysing Nitril in basic and acid condition, but I am so confused. I think better if you add some description each mecanism. Would you do it for me? thank you. please reply my questin. Thanks
BalasHapusOkay thanks via to your suggetion,i can try decribing the mechanism it,,i hope it can useful to you and other friends,but i am sorry if my describe about it less complete. And now,you can read in this under:
HapusNitrile Hydrolysis under Acidic Conditions
The cyano group has a partially negative nitrogen atom due to the higher electronegativity of the nitrogen atom. The resonance dipole of the cyano group also contributes to the negative character of the carbon atom.
The hydronium ion is a strong enough acid to react with the p bond of the cyano group…not the non-bonding electron pair on the nitrogen atom, which is sp hybridized. This yields a resonance stabilized cation.
This is one of the resonance forms of this cation and, in this form, the carbon has a positive charge and is a strong Lewis acid.
Water is a good enough nucleophile to attack strong Lewis acids such as carbocations.
N-hydrogen imines are unstable bases and will readily react with the protonated hydroxyl group (which is a strong acid) to form the resonance stabilized carbocation.
N-hydrogen imines are unstable bases and will readily react with the protonated hydroxyl group (which is a strong acid) to form the resonance stabilized carbocation.
The water will again attack the positive carbon atom, which is a strong Lewis acid.
This product is a combination of a base (the amino group) and a strong acid (the protonated alcohol group) and will readily undergo an acid base reaction…shown as an intramolecular reaction here…it can also be an intermolecular reaction.
This reaction produced a protonated amine which can act like a leaving group. An electron pair from the hydroxyl group can form an oxygen-carbon double bond and break the carbon-nitrogen bond. This will yield a resonance stabilized cation.
This step of the reaction is a rapid acid base reaction of ammonia with the protonated carboxylic acid, which is a strong acid. This reaction yields the carboxylic acid and the non-nucleophilic ammonium ion.
Nitrile Hydrolysis under Basic Conditions
The cyano group has an electropositive carbon atom due to the higher electronegativity of the nitrogen atom. The resonance dipole of the cyano group also contributes to the electropositive character of the carbon atom.
The imine anion is a strong base and will easily pull a hydrogen atom from a water molecule in the solution.
The imine product will undergo tautomerization to form an amide. This is favored by the bond energies of the reactant versus the product.
The amide carbonyl is electropositive and will be attacked by hydroxide anion. Use a double barbed arrow to show the movement of the electron pair from the hydroxide anion to the carbonyl carbon atom. Show another double barbed arrow to indicate the movement of the electrons to the oxygen atom.
The product of this reaction can reform the carbonyl and eliminate either a hydroxide anion or an amide anion. If the hydroxide ion is the leaving group, the amide is reformed. The amide anion is a much stronger base and is a very poor leaving group making this equilibrium unfavorable. A small amount can leave and this leads to the next step of the mechanism.
This step of the mechanism drives the reaction. The acid-base reaction between the carboxylic acid and the amide anion is extremely favorable and fast, which helps drive the previous equilibrium to completion.
This reaction yields the carboxylate anion and the ammonia. In a basic solution, ammonia is driven off as a gas, which also drives this reaction to completion.